Presented by GE HealthCare
Earlier this year, GE HealthCare announced Health Canada’s approval of Omnipaque injection for Oral use, making it the first and only low-osmolar iodinated contrast media approved for oral use in Canada. Omnipaque injection for Oral Use is indicated in adults and pediatrics 1.

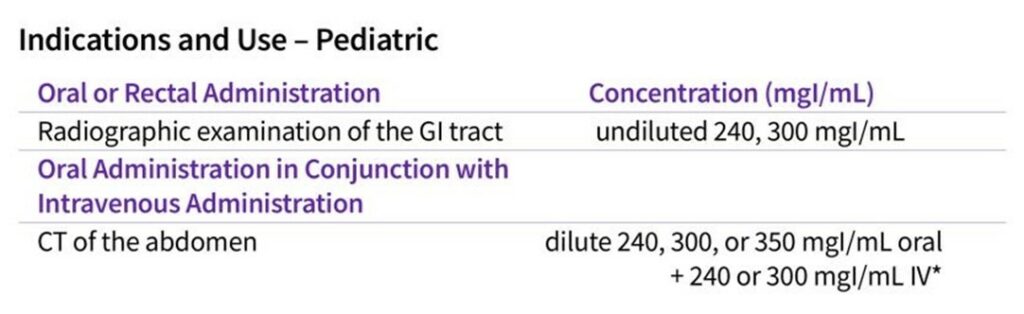
CT is utilized in imaging of the GI tract with many procedures involving administration of oral contrast agents2. For abdominal CT studies, there is often a choice between using a neutral contrast agent (such as water) or a positive oral Contrast Media2. A positive oral Contrast Media may be preferred to help improve the differentiation of the bowel from nonbowel structures on a CT scan3. The three classes of positive oral Contrast Media are barium sulfate, ionic iodinated agents, and non-ionic low-osmolar iodinated agents (e.g., Omnipaque injection for Oral Use)3.
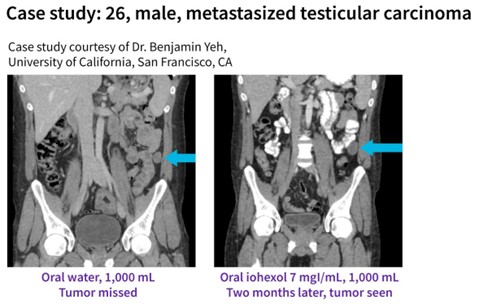
In abdominal CT, oral Contrast Media can help visualize and differentiate the structures of the bowel. Limiting their use may contribute to uncertainty in diagnoses and repeat scanning.
Recent studies have demonstrated the utility of oral Omnipaque in abdominal imaging. One study assessed the performance of Omnipaque at a reduced concentration with low-energy scanning techniques compared with barium sulfate and concluded “….with increased use of low-kVp and low-keV imaging iodinated oral contrast media (Omnipaque) has the potential for being used as a customizable LOCM, with the advantage of being a more palatable and potentially cost-saving alternative while maintaining optimal intestinal opacification in patients being treated for cancer.”4
Another study assessed the ability of Omnipaque to opacify the bowel (a determinant of image quality) in abdominopelvic CT compared with two other positive oral agents – barium sulfate and diatrizoate. It was found that “…. inhomogeneous opacification of the bowel lumen was more frequently seen with oral diatrizoate or barium sulfate than with iodinated oral contrast medium (Omnipaque). Heterogeneity in opacification in the bowel lumen may cause difficulty in CT image interpretation. Omnipaque supports image interpretation with uniform opacification in over three-quarters of bowel segments.”3
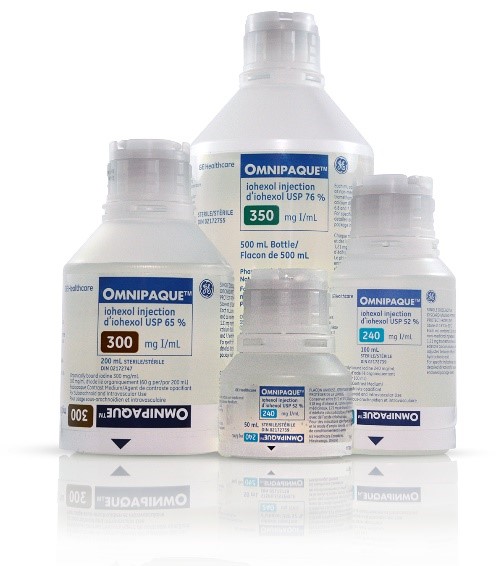
Omnipaque is provided in dilutable solution in three concentrations.
- Omnipaque 240 mg I/mL
- Omnipaque 300 mg I/mL
- Omnipaque 350 mg I/mL
Please scan the QR code below to see approved indications and Important Safety Information for Omnipaque™ (iohexol).

- CT: Computed Tomography
- GI: Gastrointestinal
- kVp: peak-kilovoltage
- keV: kiloelectron volts
- LOCM: Low Osmolar Contrast Media
References:
- Omnipaque [Product Monograph]. Mississauga, ON: GE HealthCare; 2023.
- Pickhardt PJ. Am J Roentgenol 2020; 215: 1-10..
- Winklhofer S et al. Am J Roentgenol 2019; 212(5): 1037-43.
- Parakh A et al. Radiology 2019; 291(3): 620-29
© 2024 GE HealthCare
GE is a trademark of General Electric Company used under trademark license.
March 2024 JB01045CA
